Background: Therapy choice in chronic myeloid leukemia (CML) patients (pts) with ≥2 lines of tyrosine kinase inhibitors (TKIs) failure or in pts with T315I mutationis a relevant problem. Efficacy and good tolerability of a first-in-class BCR::ABL1 allosteric inhibitor Asciminib (ASC) in chronic phase (CP) CML pts were confirmed in clinical trials. The out-study world data of ASC use in Managed Access Program (MAP) is of particular interest.
Aim: to report the updated results of the ASC use within MAP in 3 clinical centers of Russia.
Methods : Adult (≥18 years) CML pts with the lack of efficacy or intolerance to ≥2 lines of TKIs or T315I-positive pts after ≥1 prior TKI were included into a MAP. Inclusion criteria for our analysis were: CML-CP, ASC therapy for >3 months (mo). Two dosing regimen were used: 40 mg BID in BCR::ABL1 T315I-negative pts (40BID group) and 200 mg BID in BCR::ABL1 T315I-positive pts (200BID group). Complete cytogenetic response (CCyR, equivalent of MR2 or BCR::ABL1≤1%), major molecular response (MMR) and deep molecular response (MR4) achievement was assessed by cumulative incidence function (CIF) with Gray's test for comparison. Survival analysis was performed by Kaplan-Meier method according to “intention-to-treat” principle.
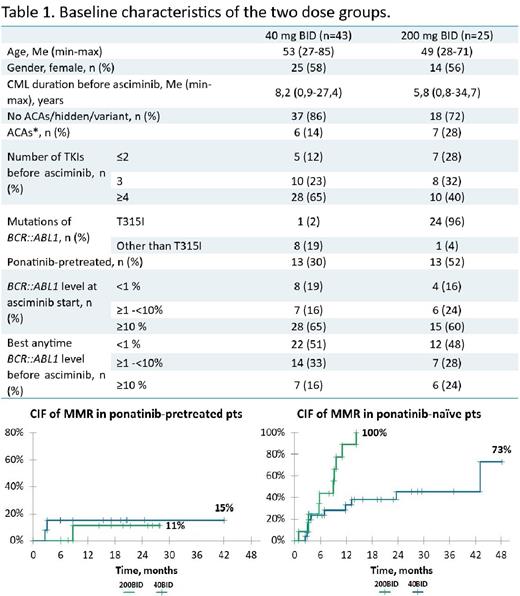
Results: A total 82 pts received ASC in the MAP, 68 of them met the criteria for analysis (baseline characteristics are in table 1), while 14 were excluded. The median follow-up was 26 mo (range 4-49).
The 2-year overall survival was 93% and 83%, the progression free survival was 90% and 70% in 40BID and 200BID groups, respectively. In 40BID group 17/43 pts discontinued ASC: 2 progressed to AP/BP, 2 - for bone marrow transplantation (BMT), 13 - failure (3 pts died due to CML after ASC discontinuation). In 200BID group 12/25 pts discontinued ASC: 2 developed AP, 3 -BMT, 7 - failure (one pt died).
CIF of CCyR/MMR/MR4 by 24 mo was 58%/68%/19% in 40BID group and 65%/56%/32% in 200BID group. The achieved CCyR was preserved in 93% (14/15) and 77% (10/13) pts in 40BID and 200BID group, respectively. CIF of MMR in ponatinib-naïve pts was higher in both groups than in ponatinib-pretreated pts (picture).
Dose escalation to 80-100 mg BID in 8/43 pts of 40BID group (due to lack of efficacy) resulted in achieving MMR for 2/8 pts with no MMR before escalation. Dose reduction in two groups was done both for adverse events (AEs) grade 3-4 (n=8) and stable MR4,5. In 4 pts (200BID) with no AEs and having a stable MR4,5 for >1 year, no MR4,5 loss was observed after ASC dose reduction to 100 mg BID.
Subclone with emerging a new mutation was detected in 3 pts of 40BID group (A337T, G250A, T315I) while no new mutations were found in 200 mg BID group.
AEs associated with ASC have been identified in 27/43 pts and 13/25 pts in 40BID and 200BID group, respectively, with no significant difference in AE spectrum and frequency. Most common (≥5%) AEs in two groups were: thrombocytopenia (29%), neutropenia (18%), cholesterol high (13%), anemia (6%), skin rash (5%). AEs grade 3-4 were detected in 13 (30%) and 4 (16%) pts in 40BID and 200BID group, respectively, and mostly presented by hematologic/cytopenic AEs (24%). No pt discontinued ASC due to toxicity, no arterial occlusive AEs were observed.
Conclusion: ASC shows effectiveness and good safety profile in pts after prior failure to TKIs and those with T315I mutation. The CIF of any response was higher in ponatinib-naive pts at any dose. Different mutational subclones evolution was observed, including emergence of myristoyl-site mutation in 1 pt. ASC half dose reduction was safe in pts with previous MR4,5 stable response.
OffLabel Disclosure:
Chelysheva:Novartis: Consultancy, Speakers Bureau; Pfizer: Speakers Bureau. Lomaia:Fusion Pharma: Speakers Bureau; Pfizer: Other: Travel, accommodation, and expenses, Speakers Bureau; Novartis: Other: Travel, accommodation, and expenses, Speakers Bureau. Morozova:Novartis: Consultancy, Speakers Bureau; Pfizer: Speakers Bureau; AMGen: Speakers Bureau. Shukhov:Novartis: Consultancy, Speakers Bureau; Pfizer: Speakers Bureau; Johnson & Johnson: Speakers Bureau; Sanofi: Speakers Bureau. Petrova:Novartis: Speakers Bureau. Vlasova:Novartis: Speakers Bureau; Pfizer: Speakers Bureau. Sbitiakova:AstraZeneca: Speakers Bureau; Novartis: Speakers Bureau. Kokhno:Novartis: Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau. Turkina:Pfizer: Other: Travel, accommodation expenses, Speakers Bureau; Fusion Pharma: Speakers Bureau; Novartis: Other: Travel, accommodation expenses, Speakers Bureau.
Dose reduction of asciminib from 200 mg BID to 200 mg QOD in patients with stable deep molecular response

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal